CRITICAL CARE TRIALS
Stay ahead of the curve with exciting new clinical trials from the critical care field presented in vivid, visual abstract format. Gain comprehensive and insightful perspectives as each critical development is delivered to you.
TTM2 Trial
Jun 17, 2021
Hypothermia versus Normothermia after Out-of-Hospital Cardiac Arrest.
In a multicenter RCT of 1861 comatose survivors of out-of-hospital cardiac arrest, targeted hypothermia at 33°C did not significantly reduce 6-month all-cause mortality compared to targeted normothermia with fever control (50% vs. 48%; P = 0.37). Functional outcomes were also similar (poor mRS: 55% vs. 55%). Hypothermia increased arrhythmias (24% vs. 17%; P < 0.001). These findings emphasize the importance of active temperature management and hyperthermia avoidance rather than hypothermia itself.
MENDS2 Trial
Apr 15, 2021
Dexmedetomidine or Propofol for Sedation in Mechanically Ventilated Adults with Sepsis.
In a multicenter RCT of 432 mechanically ventilated septic patients, dexmedetomidine did not improve days alive without delirium or coma compared to propofol (median 10.7 vs. 10.8 days). Ventilator-free days (23.7 vs. 24.0), 90-day mortality (38% vs. 39%), and 6-month cognitive outcomes (TICS-T scores: 40.9 vs. 41.4) were similar. Safety profiles were comparable. Findings support continued use of either sedative based on clinical preference or patient-specific factors.
HOT-ICU Trial
Apr 8, 2021
Lower or Higher Oxygenation Targets for Acute Hypoxemic Respiratory Failure.
In a multicenter RCT of 2928 ICU patients with acute hypoxemic respiratory failure, targeting a lower PaO₂ (60 mm Hg) did not reduce 90-day mortality compared to a higher target (90 mm Hg) (42.9% vs. 42.4%; P = 0.64). Secondary outcomes, including days alive without life support and ischemic events, were also similar. Findings support maintaining PaO₂ targets within the range of 60–90 mm Hg without favoring a lower target for improved outcomes.
AKIKI2 Trial
Apr 3, 2021
Comparison of two delayed strategies for renal replacement therapy initiation for severe acute kidney injury (AKIKI 2): a multicentre, open-label, randomized, controlled trial.
In a multicenter RCT of critically ill patients with severe AKI (KDIGO 3), a more-delayed RRT strategy (initiated only upon mandatory indications or BUN >140 mg/dL) did not increase RRT-free days compared to a delayed strategy (BUN >112 mg/dL or >72 hours oliguria) (median 12 days for both; P = 0.93). Mortality at 60 days was higher with the more-delayed strategy (HR 1.65, 95% CI 1.09–2.50, P = 0.018). Early RRT initiation within 72 hours is recommended.
HALT-IT
Jun 20, 2020
Effects of a high-dose 24-h infusion of tranexamic acid on death and thromboembolic events in patients with acute gastrointestinal bleeding
This multicenter RCT evaluated high-dose 24-hour tranexamic acid infusion versus placebo in 11,937 adults with acute gastrointestinal bleeding. Death from bleeding within 5 days was similar (3.7% vs. 3.8%; RR 0.99, 95% CI 0.82–1.18). Tranexamic acid increased venous thromboembolic events (0.8% vs. 0.4%; RR 1.85, 95% CI 1.15–2.98) and seizures (0.6% vs. 0.4%; RR 1.73, 95% CI 1.03–2.93). Secondary outcomes, including rebleeding and all-cause mortality, were comparable. Findings indicate no mortality benefit with tranexamic acid and highlight safety concerns, warranting further subgroup analyses.
NONSEDA Trial
Mar 19, 2020
Nonsedation or Light Sedation in Critically Ill, Mechanically Ventilated Patients.
In a multicenter RCT, no sedation did not reduce 90-day mortality compared to light sedation (42.4% vs. 37.0%; P = 0.65) in mechanically ventilated patients. Secondary outcomes, including ventilator-free and ICU-free days, were similar. Non-sedation reduced thromboembolic events (0.3% vs. 2.8%) but increased accidental extubations (8.9% vs. 4%). These findings support targeting light sedation (RASS -2 to 1) with daily interruptions rather than no sedation
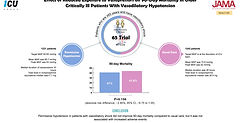
65 Trial
Feb 12, 2020
Effect of Reduced Exposure to Vasopressors on 90-Day Mortality in Older Critically Ill Patients With Vasodilatory Hypotension.
In a multicenter RCT of 2463 elderly patients with vasodilatory shock, targeting permissive hypotension (MAP 60–65 mmHg) did not significantly reduce 90-day mortality compared to standard care (41% vs. 43.8%; ARD −2.85%, 95% CI −6.75 to 1.05; P = 0.15). Vasopressor exposure was lower in the permissive hypotension group. Subgroup analysis suggested potential mortality benefit in patients with chronic hypertension. Permissive hypotension appears safe and may be a reasonable approach in elderly patients.
DEXA-ARDS
Feb 7, 2020
Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial.
In a multicenter RCT of 277 patients with moderate to severe ARDS, dexamethasone significantly increased ventilator-free days (12.3 vs. 7.5 days; P < 0.0001) and reduced 60-day mortality (21% vs. 36%; P = 0.0047; NNT = 7) compared to standard care. Adverse events were similar between groups. Findings support dexamethasone use in ARDS, especially cases related to sepsis or pneumonia. Further research is needed for ARDS of other etiologies, such as trauma.
HYPERION Trial
Dec 12, 2019
Targeted Temperature Management for Cardiac Arrest with Nonshockable Rhythm.
In a multicenter RCT of 581 comatose survivors of cardiac arrest due to non-shockable rhythm, moderate hypothermia (33°C) significantly improved 90-day survival with favorable neurological outcomes compared to normothermia (10.2% vs. 5.7%; P = 0.04; NNT = 22). Ninety-day mortality (81.3% vs. 83.2%) and adverse events were similar between groups. These findings support the use of therapeutic hypothermia in non-shockable cardiac arrest patients to improve neurologic outcomes.
ROSE Trial
May 23, 2019
Early Neuromuscular Blockade in the Acute Respiratory Distress Syndrome.
In a multicenter RCT of 1006 patients with moderate to severe ARDS (PaO₂/FiO₂ <150), 48-hour continuous cisatracurium infusion with deep sedation did not reduce 90-day mortality compared to usual care with lighter sedation (42.5% vs. 42.8%; P = 0.93). Secondary outcomes, including ventilator-free and ICU-free days, were also similar. Routine neuromuscular blockade is not supported and should be reserved for select cases, such as ventilator asynchrony or high respiratory effort.
PREVENT Trial
May 4, 2019
Adjunctive Intermittent Pneumatic Compression for Venous Thromboprophylaxis.
In a multicenter RCT of 2003 critically ill adults, adding intermittent pneumatic compression to pharmacologic thromboprophylaxis did not significantly reduce proximal deep vein thrombosis incidence compared to pharmacologic thromboprophylaxis alone (3.9% vs. 4.2%; P = 0.74). Secondary outcomes, including venous thromboembolism and 90-day mortality, were also similar. Findings do not support routine use of pneumatic compression in ICU patients receiving anticoagulant prophylaxis.
ANDROMEDA-SHOCK Trial
Feb 17, 2019
Effect of a Resuscitation Strategy Targeting Peripheral Perfusion Status vs Serum Lactate Levels on 28-Day Mortality Among Patients With Septic Shock.
In a multicenter RCT of 424 patients with septic shock, targeting capillary refill time for resuscitation did not significantly reduce 28-day mortality compared to lactate-targeted resuscitation (34.9% vs. 43.4%; P = 0.06). Capillary refill-targeted resuscitation improved organ dysfunction at 72 hours (SOFA score 5.6 vs. 6.6; P = 0.045). Secondary outcomes, including mechanical ventilation- and vasopressor-free days, were similar. Findings suggest capillary refill targeting is a safe alternative but does not clearly improve mortality.