CRITICAL CARE TRIALS
Stay ahead of the curve with exciting new clinical trials from the critical care field presented in vivid, visual abstract format. Gain comprehensive and insightful perspectives as each critical development is delivered to you.
Dec 7, 2025
RSI Trial
Ketamine or Etomidate for Tracheal Intubation of Critically Ill Adults
In this multicenter RCT (n=2,365), critically ill adults undergoing emergency tracheal intubation were randomized to ketamine (n=1176) or etomidate (n=1189) for anesthesia induction. In-hospital mortality by day 28 was 28.1% with ketamine and 29.1% with etomidate (adjusted risk difference −0.8 percentage points; 95% CI, −4.5 to 2.9; P=0.65). Cardiovascular collapse during intubation occurred more frequently with ketamine (22.1% vs 17.0%; risk difference 5.1 percentage points; 95% CI, 1.9 to 8.3), particularly in patients with sepsis or high illness severity. No significant differences were found in secondary outcomes including ICU-free days, vasopressor-free days, and 24-hour BP readings.
9
Nov 10, 2025
BICARICU-2 Trial
Sodium Bicarbonate for Severe Metabolic Acidemia and Acute Kidney Injury
In the BICARICU-2 trial, critically ill patients with severe metabolic acidemia (pH ≤ 7.20, bicarbonate ≤ 20 mEq/L, PaCO₂ ≤ 45 mm Hg) and moderate to severe acute kidney injury (AKI stage 2–3 by KDIGO) admitted to 43 French ICUs were randomized to receive intravenous sodium bicarbonate infusion targeting arterial pH ≥ 7.30 (intervention) or no bicarbonate therapy with standard care (comparison). The primary outcome was 90-day all-cause mortality, with secondary outcomes including mortality at 28 and 180 days, renal replacement therapy (RRT) use and timing, organ support duration, and ICU or hospital stay. The main results showed no mortality difference between groups (62.1% vs 61.7%), but sodium bicarbonate was associated with a lower rate and delayed initiation of RRT (35% vs 50%, median 30.9 h vs 15.5 h). The conclusion was that intravenous sodium bicarbonate did not improve survival but appeared safe and may reduce or postpone the need for RRT in severe acidemia with AKI.
208
Nov 8, 2025
The TOP Trial
Liberal or Restrictive Postoperative Transfusion in Patients at High Cardiac Risk
The TOP trial enrolled 1,428 postoperative patients with high baseline cardiac risk—including those with ischemic heart disease, myocardial infarction, stroke, transient ischemic attack, or peripheral artery disease—undergoing major vascular or general surgery at 16 Veterans Affairs hospitals. Participants were randomized to receive red blood cell transfusions using either a liberal strategy (initiated at hemoglobin <10 g/dL) or a restrictive strategy (<7 g/dL). The primary outcome, a 90-day composite of all-cause mortality, myocardial infarction, ischemic stroke, coronary revascularization, or acute kidney injury, occurred in 9.1% of the liberal group and 10.1% of the restrictive group (relative risk 0.90; 95% CI, 0.65–1.24), showing no statistically significant difference. However, secondary analysis revealed that cardiac complications excluding myocardial infarction—such as arrhythmias, new or worsening heart failure, or nonfatal cardiac arrest—were significantly more frequent in the restrictive group (9.9% vs. 5.9%; relative risk 0.59; 99% CI, 0.36–0.98), suggesting potential benefit from a liberal transfusion approach in this high-risk population.
11
Nov 7, 2025
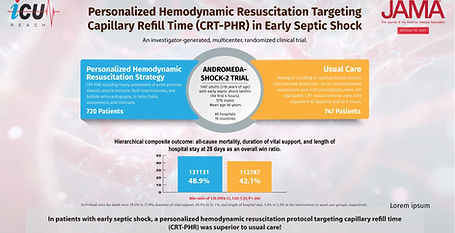
ANDROMEDA-SHOCK-2
Personalized Hemodynamic Resuscitation Targeting Capillary Refill Time in Early Septic Shock
The ANDROMEDA-SHOCK-2 trial, published in JAMA (2025), represents a major evolution in early septic shock management. Across 86 intensive care units in 19 countries, researchers compared a personalized hemodynamic resuscitation strategy targeting capillary refill time (CRT-PHR) with usual care. Among 1467 analyzed patients, CRT-guided therapy demonstrated superiority on a hierarchical composite outcome of 28-day mortality, duration of vital support, and length of hospital stay (win ratio 1.16; 95% CI, 1.02–1.33; P = .04). The benefit was driven primarily by fewer days requiring vasopressors, mechanical ventilation, or kidney replacement therapy.
11
Nov 6, 2025
EVERDAC Trial
Deferring Arterial Catheterization in Critically Ill Patients with Shock
In this multicenter noninferiority trial involving ICU patients with shock, early arterial catheter placement was compared to initial noninvasive blood pressure monitoring using an automated cuff. The study found that deferring arterial catheterization did not increase 28-day mortality (34.3% in the noninvasive group vs. 36.9% in the invasive group; adjusted risk difference −3.2%; P=0.006 for noninferiority). Additionally, patients in the noninvasive group experienced fewer catheter-related complications such as hematoma or bleeding (1.0% vs. 8.2%). These findings suggest that early arterial line insertion may not be necessary in all patients with shock and that a noninvasive approach can be safely considered, reserving invasive monitoring for those who meet specific clinical criteria.
508
Oct 29, 2025
TARGET Protein Trial
Augmented Enteral Protein During Critical Illness
In a cluster randomized crossover trial across 8 ICUs (n=3397), augmented enteral protein (100 g/L) did not improve clinical outcomes compared to usual protein (63 g/L). The primary outcome—days alive and free from hospitalization at day 90—showed no significant difference (median 62 vs 64 days; adjusted median difference −1.97 days; 95% CI, −7.24 to 3.30; P=.46). Ninety-day survival was similar (72.6% vs 74.0%; RR 0.99; 95% CI, 0.95–1.03). No differences were observed in hospital-free days among survivors, invasive ventilation duration, ICU or hospital stay lengths, tracheostomy rates, or new kidney replacement therapy. Discharge destinations were comparable. Augmented protein did not confer clinical benefit in critically ill adults.
13
Apr 2, 2025
REMAP-CAP
Effect of hydrocortisone on mortality in patients with severe community-acquired pneumonia
In an international adaptive randomized controlled trial involving ICU patients with severe community-acquired pneumonia (CAP), a fixed 7-day course of intravenous hydrocortisone (50 mg every 6 hours) was compared to no corticosteroid. Among 658 enrolled patients (536 hydrocortisone, 122 control), 90-day mortality was 15% in the hydrocortisone group and 9.8% in the control group, with adjusted odds ratios ranging from 1.52 to 1.63 (95% CI all crossing 1). The probability of achieving a >20% relative mortality reduction with hydrocortisone was low (3.3–7.1%), and the trial arm was stopped early for futility. Secondary and sensitivity analyses showed consistent results, though an exploratory analysis suggested a modest reduction in shock duration with hydrocortisone (median 2 vs. 3 days, p=0.05). Overall, hydrocortisone did not confer a mortality benefit in severe CAP and may carry potential for harm, indicating it should not be routinely used in this context. Results should be interpreted with caution and taking into account the meta-analysis that was published later.
2
Mar 12, 2025
BALANCE Trial
Antibiotic Treatment for 7 versus 14 Days in Patients with Bloodstream Infections
The BALANCE trial, published in The New England Journal of Medicine in 2025, investigated whether a 7-day course of antibiotics was as effective as 14 days in hospitalized adults with bloodstream infections, including critically ill ICU patients. Conducted across 74 hospitals in seven countries with over 3,600 participants, the randomized, noninferiority trial excluded patients with Staphylococcus aureus, severe immunosuppression, or infections requiring prolonged therapy. Mortality at 90 days was 14.5% in the 7-day group and 16.1% in the 14-day group (difference, −1.6 percentage points; 95.7% CI, −4.0 to 0.8), confirming noninferiority of the shorter regimen. Rates of relapse, Clostridioides difficile infection, and antibiotic resistance were similar between groups, demonstrating that a 7-day antibiotic course is safe, effective, and stewardship-aligned for most stabilized patients with bloodstream infection.
2
Dec 18, 2024
OPTIMISE Trial
Seven versus 14 days of antimicrobial therapy for severe multidrug-resistant Gram-negative bacterial infections in intensive care unit patients
This trial evaluated the non-inferiority of 7-day versus 14-day antimicrobial therapy for ICU-acquired severe infections caused by MDR-GNB. In the ITT population (n=106), clinical failure rates were 42.4% (7-day) and 44.7% (14-day) (RD −2.3%, 95%CI −21.3 to 16.7). The trial was underpowered due to early termination from low recruitment, leaving non-inferiority inconclusive. Respiratory tract infections were most common (68.9%), often caused by carbapenem-resistant Enterobacterales.
7
Oct 30, 2024
PEERLESS Trial
Large-bore Mechanical Thrombectomy Versus Catheter-directed Thrombolysis in the Management of Intermediate-risk Pulmonary Embolism
The PEERLESS trial compared large-bore mechanical thrombectomy (LBMT) to catheter-directed thrombolysis (CDT) in intermediate-risk PE. Among 550 patients, LBMT significantly reduced the composite primary endpoint (win ratio 5.01, 95% CI: 3.68–6.97, p<0.001) by lowering rates of clinical deterioration, ICU admissions (41.6% vs. 98.6%), and prolonged ICU stays (>24 hours: 19.3% vs. 64.5%). Mortality and major bleeding rates were similar, but LBMT improved respiratory outcomes and shortened hospital stays (4.5 vs. 5.3 days, p=0.002). These findings highlight LBMT as a superior intervention for intermediate-risk PE.
147
Aug 17, 2024
PRECISe
Effect of high versus standard protein provision on functional recovery in people with critical illness
This multicenter RCT compared high protein (2.0 g/kg/day) to standard protein (1.3 g/kg/day) provision in 935 mechanically ventilated critically ill patients. High protein was associated with worse health-related quality of life at 30, 90, and 180 days post-randomization (mean EQ-5D-5L score difference: -0.05; 95% CI -0.10 to -0.01; p=0.031). Mortality rates were similar, but gastrointestinal intolerance was higher with high protein (OR 1.76; 95% CI 1.06–2.92; p=0.030). Findings favor standard protein provision for better recovery and fewer complications
176
Jun 14, 2024
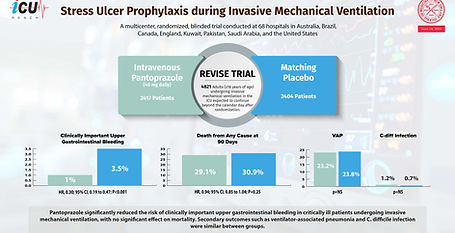
REVISE TRIAL
Stress Ulcer Prophylaxis during Invasive Mechanical Ventilation
This international, blinded RCT evaluated pantoprazole (40 mg daily) versus placebo in 4821 mechanically ventilated ICU patients. Pantoprazole significantly reduced clinically important upper gastrointestinal bleeding (1.0% vs. 3.5%; HR 0.30, 95% CI 0.19–0.47, p<0.001) and patient-important bleeding (1.5% vs. 4.2%; HR 0.36, 95% CI 0.25–0.53, p<0.001). There was no effect on 90-day mortality (29.1% vs. 30.9%; HR 0.94, p=0.25). Rates of ventilator-associated pneumonia and C. difficile infection were similar. Pantoprazole effectively prevents gastrointestinal bleeding without impacting overall survival.
463