CRITICAL CARE TRIALS
Stay ahead of the curve with exciting new clinical trials from the critical care field presented in vivid, visual abstract format. Gain comprehensive and insightful perspectives as each critical development is delivered to you.
Mar 20, 2023
NUTRIREA-3
Low versus standard calorie and protein feeding in ventilated adults with shock
This multicenter RCT evaluated early low-calorie, low-protein nutrition (6 kcal/kg/day, 0.2–0.4 g/kg/day protein) versus standard targets (25 kcal/kg/day, 1.0–1.3 g/kg/day) in 3036 ICU patients receiving mechanical ventilation and vasopressors for shock. Mortality at 90 days was similar (41.3% vs. 42.8%; p=0.41), but time to ICU discharge readiness was faster in the low group (median 8.0 vs. 9.0 days; HR 1.12, p=0.015). The low group had fewer gastrointestinal complications. Findings suggest a restrictive nutrition strategy may expedite recovery without affecting survival.
310
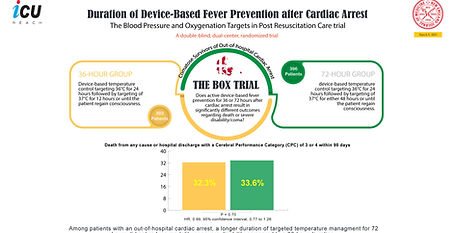
Mar 9, 2023
BOX Trial (Duration)
Duration of Device-Based Fever Prevention after Cardiac Arrest
In a randomized trial of comatose patients resuscitated after out-of-hospital cardiac arrest, 36-hour vs. 72-hour device-based fever prevention showed no significant differences in mortality or severe disability/coma. Limitations included unmasked interventions and modest fever prevention efficacy. The results suggest no added benefit to extending device-based fever prevention beyond 36 hours
120
Jan 21, 2023
The CLOVERS Trial
Early Restrictive or Liberal Fluid Management for Sepsis-Induced Hypotension
In a multicenter trial of 1563 patients with sepsis-induced hypotension, restrictive fluid strategy (early vasopressor use, minimal fluids) showed no significant difference in 90-day mortality compared to liberal fluid strategy (fluid-predominant approach) (14.0% vs. 14.9%; P = 0.61). Secondary outcomes were also similar. Restrictive fluid strategy was safe, with few complications. Results suggest either approach is viable, but findings are limited to early sepsis management.
651
Nov 17, 2022
Spontaneous Breathing Trials
Spontaneous-Breathing Trials with Pressure-Support Ventilation or a T-Piece
In a multicenter trial of 969 high-risk extubation failure patients, spontaneous-breathing trials with PSV did not significantly increase ventilator-free days at day 28 compared to T-piece trials (median 27 days for both; P = 0.31). Extubation and reintubation rates were similar between groups. Findings suggest that either PSV or T-piece can be used for spontaneous-breathing trials in high-risk patients, with outcomes influenced by prophylactic noninvasive ventilation practices.
183
Oct 26, 2022
AID-ICU
Haloperidol for the Treatment of Delirium in ICU Patients
In a multicenter RCT of 1000 ICU patients with delirium, haloperidol did not significantly improve days alive and out of the hospital at 90 days compared to placebo (mean 35.8 vs. 32.9 days; adjusted difference 2.9 days; 95% CI -1.2 to 7.0; P = 0.22). Mortality was lower but not statistically significant (36.3% vs. 43.3%). Serious adverse reactions were similar. Haloperidol may aid in managing hyperactive delirium but does not improve long-term outcomes.
138
Oct 24, 2022
The PILOT Trial
Oxygen-Saturation Targets for Critically Ill Adults Receiving Mechanical Ventilation
In a single-center, cluster-randomized trial of 2541 mechanically ventilated adults, there was no significant difference in ventilator-free days at day 28 between lower (88–92%), intermediate (92–96%), or higher (96–100%) oxygen-saturation targets (median 20 vs. 21 vs. 21 days; P = 0.81). Mortality and adverse events were similar across groups. The findings suggest no clear benefit or harm from varying oxygen saturation targets, supporting flexibility in clinical practice.
127
Sep 28, 2022
HACA-IHCA
Temperature Control After In-Hospital Cardiac Arrest: A Randomized Clinical Trial
In a multicenter trial of 249 comatose patients after in-hospital cardiac arrest (IHCA), hypothermic temperature control (32–34°C) did not significantly improve 180-day mortality (72.5% vs. 71.2%; RR 1.03, 95% CI 0.79–1.40, P = 0.822) or functional outcomes (22.5% vs. 23.7%; RR 1.04, 95% CI 0.78–1.44, P = 0.822) compared to normothermia (36–37.5°C). While the study was underpowered, it supports maintaining normothermia and avoiding fever in post-IHCA care.
100
Sep 15, 2022
WATERFALL Trial
Aggressive or Moderate Fluid Resuscitation in Acute Pancreatitis
In a multicenter RCT of 249 patients with acute pancreatitis, aggressive fluid resuscitation did not significantly reduce rates of moderately severe or severe pancreatitis (22.1% vs. 17.3%; RR 1.30, 95% CI 0.78–2.18, P = 0.32) but was associated with higher fluid overload risk (20.5% vs. 6.3%; RR 2.85, 95% CI 1.36–5.94, P = 0.004). The trial was terminated early for safety concerns. Moderate fluid resuscitation is safer and recommended in this population.
732
Aug 27, 2022
BOX Trial (MAP)
Blood-Pressure Targets in Comatose Survivors of Cardiac Arrest
In a randomized trial of 789 comatose survivors of out-of-hospital cardiac arrest, targeting a higher MAP (77 mm Hg) did not significantly improve the composite outcome of death or severe disability (34% vs. 32%; HR 1.08, 95% CI 0.84–1.37, P = 0.56) compared to a lower MAP (63 mm Hg). Mortality, functional outcomes, and adverse events were similar. Findings suggest no benefit in targeting MAP >65 mm Hg in post-cardiac arrest care.
94
Aug 27, 2022
BOX Trial (Oxygenation)
Oxygen Targets in Comatose Survivors of Cardiac Arrest
In a randomized trial of 789 comatose adults after out-of-hospital cardiac arrest, a restrictive oxygenation target (Pao2 68–75 mm Hg) did not significantly improve the composite outcome of mortality or severe disability/coma at 90 days compared to a liberal target (Pao2 98–105 mm Hg) (32% vs. 33.9%; HR 0.95, 95% CI 0.75–1.21, P = 0.69). Mortality, functional scores, and adverse events were similar. Findings suggest no benefit to restrictive oxygen targets in this setting.
66
Jul 6, 2022
Oral Sabizabulin for COVID-19
Oral Sabizabulin for High-Risk, Hospitalized Adults with Covid-19: Interim Analysis
In a multicenter RCT of 204 high-risk hospitalized adults with moderate to severe COVID-19, oral Sabizabulin significantly reduced 60-day all-cause mortality compared to placebo (20.2% vs. 45.1%; OR 3.23, 95% CI 1.45–7.22, P = 0.0042; NNT = 4). Sabizabulin also reduced ICU days (43%), ventilator days (49%), and hospital days (26%). Benefits were consistent across subgroups. Sabizabulin demonstrates strong efficacy and safety in preventing COVID-19 progression to ARDS and death.
20
Jul 5, 2022
The COVIDICUS Randomized Clinical Trial
High-Dose Dexamethasone and Oxygen Support Strategies in Intensive Care Unit Patients With Severe COVID-19 Acute Hypoxemic Respiratory Failure.
In a multicenter trial of 841 patients with severe COVID-19 and hypoxemic respiratory failure, high-dose dexamethasone did not reduce 60-day mortality compared to standard-dose dexamethasone (HR 0.96, 95% CI 0.69–1.33, P = 0.79). Among 333 non-intubated patients, respiratory support strategies (O₂ therapy, CPAP, or HFNO₂) showed no significant difference in 28-day invasive mechanical ventilation rates or 60-day mortality. Standard dexamethasone and patient-preferred oxygen strategies remain recommended.
45