Neurological Prognostication after Cardiac Arrest: What Every Intensivist Should Know!
- Mazen Kherallah

- Nov 5, 2022
- 5 min read
Updated: Apr 9, 2023

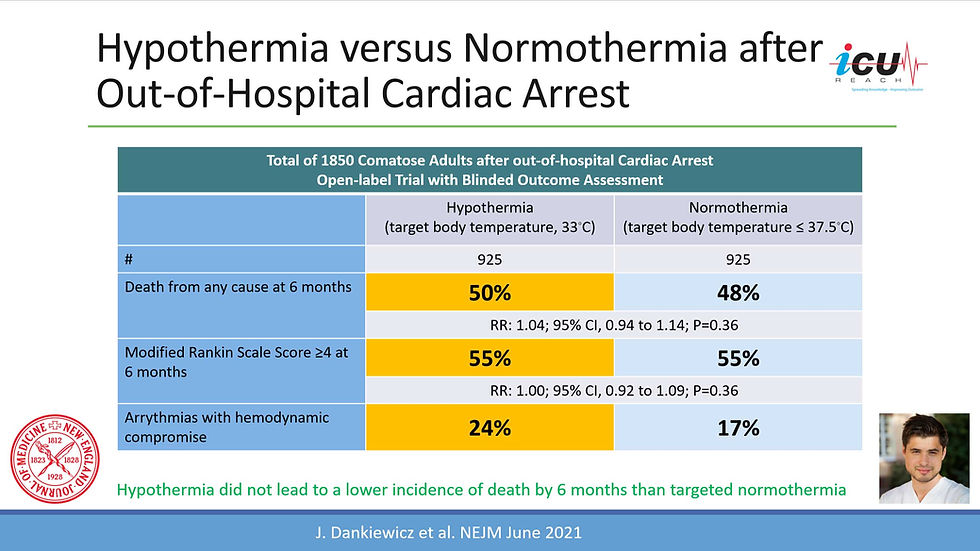
Even though care for post-cardiac arrest patients has improved over the past 20 years since the inception of therapeutic temperate management many patients who have had cardiac arrests remain comatose after being rewarmed from targeted temperature management (TTM) and sedation discontinuation. While the care of post-cardiac arrest patients has improved over the past two decades since therapeutic temperate management began, many patients remain comatose after being rewarmed from targeted temperature management and sedation discontinuation. A decision to withdraw life-sustaining treatment should only be based on precise and accurate prognostic indicators to avoid falsely pessimistic predictions. However, there is no single indicator that is highly specific and accurate for the postanoxic comatose state. Therefore, the clinician should rely on a multimodal approach in which the decision is based on clinical examination, neurophysiology testing (electroencephalography, somatosensory evoked potentials), biomarkers (neuron-specific enolase and S-100β), and brain imaging (computed tomography scan and magnetic resonance imaging).
The European Resuscitation Council (ERC) and the European Society of Intensive Care Medicine (ESICM) have collaborated to produce post-resuscitation care guidelines for adults who have suffered cardiac arrest. These guidelines included a roadmap for neuroprognostication that informs the patient’s relatives about the patient’s chances of achieving a neurologically meaningful recovery, as well as helping clinicians target treatments based on prognosis.
These guidelines recommend that in comatose patients with a Glasgow Motor Score of ≤ 3 at ≥ 72 h from the return of spontaneous circulation (ROSC), in the absence of confounders, a poor outcome is likely when two or more of the following predictors are present:
No pupillary and corneal reflexes at ≥ 72 h
Bilaterally absent N20 SSEP wave at ≥ 24 h
Highly malignant EEG at > 24 h
Neuron-specific enolase (NSE) > 60 µg/L at 48 h and/or 72 h
Status myoclonus ≤ 72 h
Diffuse and extensive anoxic injury on brain CT/MRI
It is important to mention that a final prognostic assessment should only be undertaken when the patient is rewarmed after TTM and all confounding factors have been ruled out. Most of these signs can be recorded before 72 h from ROSC; however, their results will be evaluated only at the time of clinical prognostic assessment.
What is the best time to determine neurological prognosis after cardiac arrest?
24 hours after ROSC
48 hours after ROSC
72-96 hours after ROSC
Clinical examination
The neurological examination prognostication is largely dependent on the response to painful stimuli, pupillary reaction, corneal reflex, and gag and cough reflexes. To ensure an accurate assessment, it's important that clinicians rule out any confounding factors from sedatives or other drugs (opioids or muscle relaxants) that may alter the results of the examination. Additionally, pay attention to any metabolic conditions as they can also have an impact on the outcome of the clinical assessment.
A Glasgow Motor Score of ≤ 3 (abnormal flexion or worse in response to pain) between 24-72 hours after ROSC is worrisome but nonspecific. At 72 h or later, the score may identify patients at high risk of poor neurological prognosis and a full assessment may be needed.
Pupillary reflexes are fairly resistant to the effects of sedatives or paralysis. However, their absence in the first 72 hours after ROSC is worrisome but yet nonspecific. The specificity of the examination is ~99% at 72 hours and approaches 100% at 96%. Quantitative pupillometry has superior performance and may have a higher predictive value early in the patient's course but it is not widely available.
The absence of corneal reflexes at 72 hours has a specificity of ~95% specific for a poor neurologic outcome and increases to approach 100% at 96 hours. Though not well-studied, the absence of a gag or cough reflex has shown to be reliable 48 hours or more after ROSC.
Finally, the presence of myoclonus within 96 hours and, in particular, the presence of status myoclonus (generalized synchronized, repetitive, symmetric, and unequivocal movements in four limbs and the face within 72 hours, persisting for more than 30 minutes, and associated with EEG findings of seizure or a malignant pattern) may indicate a poor prognosis.
Neurophysiology
The timing of various EEG patterns is crucial. The most opportune single time point to evaluate an EEG seems to be around 24 hours after arrest. Beyond that, the EEG will usually improve with time, thereby decreasing its sensitivity to detect patients who will have a poor neurological prognosis. For an optimal recording, medications (e.g., propofol) that can affect the EEG should be withheld for several half-lives. Motor artifacts, like myoclonus, shivering, and respiration can make the EEG difficult to interpret. To get a high-quality EEG in comatose patients, we may need to paralyze the patient temporarily (paralysis without sedation is acceptable only in a comatose patient).
The EEG is a combination of the background pattern and any superimposed activity. The background pattern is the most important aspect of the EEG. Continuous background activity occurring within the first 60 hours post-ROSC is an indication of a favorable outcome, while a discontinuous background (periods of suppression of 10-40% of the time) is nonspecific and requires further monitoring and investigations. Highly malignant EEG patterns include suppressed background (low amplitude of <10 µV in >99% of the time) with or without periodic discharges, and burst suppression (bursts of EEG activity on a flat background more than 50% of the time). The presence of unequivocal seizures on EEG during the first 72 h after ROSC is an indicator of a poor prognosis
The somatosensory evoked cortical N20-potentials (negative peak at 20 ms) are measured non-invasively by transcutaneous stimulation of the median nerve at the wrist and detecting the cortical somatosensory electrical signal by an electrode placed over the contralateral skull. The bilateral absence of somatosensory evoked cortical N20-potentials more than 24 hours after ROSC is an indicator of poor prognosis and has a sensitivity of ~45% and a specificity of >99%.
Biomarkers
Neuron-specific enolase (NES) is an enzyme released after brain injury or hemolysis. Its level is not significantly affected by targeted temperature management at 33°C or 36°C. Elevated (higher than 60 ng/ml ) or rising levels between 24 and 48 h or 72 h predict a poor neurological outcome. The test is not widely used due to the lack of availability, absence of calibration standards, and disagreement regarding the optimal cutoff.
Imaging
Studies have shown that loss of gray-white matter differentiation on a computed tomography (CT) scan of the brain is a reliable predictor of poor outcomes with a false potitve rate of zero in multiple studies. However, the sensitivity of these findings remains very low. In most cases of cardiac arrest, CT scan is done on admission to rule out other causes of cardiac arrest and in rare cases, it might show evidence of anoxic injury on presentation. However, the optimal time window to obtain a CT scan is at 72 hours after ROSC as edema may become more prominent over ~3-5 days following cardiac arrest. MRI is more sensitive than CT and if available, it would be the preferred imaging study to determine the presence of anoxic injury. Abnormalities on diffusion and apparent diffusion coefficient sequence of MRI can be positive as early as 48 hours post cardiac arrest and the optimal window is probably between 2-5 days after ROSC.
Summary
Determination of poor neurological prognosis after cardiac arrest can be best done at least 72 hours after the return of spontaneous circulation. A multimodal approach in which the decision is based on clinical examination, neurophysiology testing (electroencephalography, somatosensory evoked potentials), biomarkers (neuron-specific enolase), and brain imaging (computed tomography scan and magnetic resonance imaging) can increase the reliability of the assessment and help to make an appropriate prognostication for the patient's decision maker to be informed.
References
Nolan JP, Sandroni C, Böttiger BW, Cariou A, Cronberg T, Friberg H, Genbrugge C, Haywood K, Lilja G, Moulaert VRM, Nikolaou N, Olasveengen TM, Skrifvars MB, Taccone F, Soar J. European Resuscitation Council and European Society of Intensive Care Medicine guidelines 2021: post-resuscitation care. Intensive Care Med. 2021 Apr;47(4):369-421. doi: 10.1007/s00134-021-06368-4. Epub 2021 Mar 25. PMID: 33765189; PMCID: PMC7993077.
Sandroni, C., D’Arrigo, S. & Nolan, J.P. Prognostication after cardiac arrest. Crit Care22, 150 (2018). https://doi.org/10.1186/s13054-018-2060-7



Comments